Health Alert: Unregulated Botox Injections Under Scrutiny After Botulism Cases
The Biden administration is undertaking a multi-state inquiry into ailments that may be linked to botulinum toxin injections, better known as Botox, in at least two states.
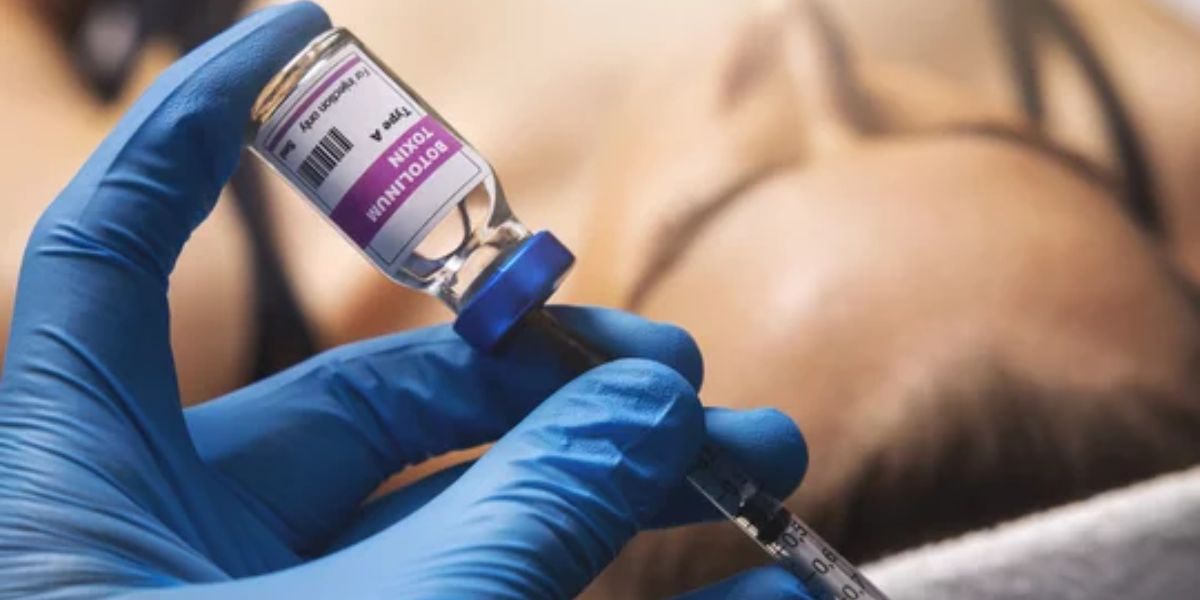
A spokeswoman for the Centers for Disease Control and Prevention (CDC) verified to The Hill that the Botox injections were performed in “non-medical settings,” and the source of the items remains unknown.
The CDC is collaborating with health agencies in Tennessee and Illinois, where Botulism-like symptoms have been recorded in numerous patients, according to a spokesman.
According to the CDC, botulism is a dangerous sickness caused by the toxin Clostridium botulinum, which targets the body’s nerves. Symptoms often include weakness in the eye, face, and throat muscles, which can progress to the neck, arms, body, and legs. It can also weaken one’s muscles, making breathing difficult and, in certain cases, fatal.
According to the CDC, antitoxins are frequently used to treat the condition.
Botox injections contain minuscule amounts of the toxins at levels authorized and approved by the Food and Drug Administration (FDA).
Illinois health officials cautioned healthcare facilities and hospital emergency rooms this week to be on the lookout for symptoms after two cases were reported of patients receiving Botox injections, or a “similar, possibly counterfeit product.”
The Illinois Department of Public Health reported that the two individuals had botulism-like symptoms such as blurred or double vision, a droopy face, exhaustion, shortness of breath, difficulty breathing, and a raspy voice. Both victims were hospitalized and given injections by a licensed nurse in LaSalle County, Illinois, “who was performing work outside her authority,” according to the department.
The Tennessee Department of Health stated last week that four patients developed botulism-like symptoms. State health officials reported that their investigations with the CDC and FDA have highlighted concerns regarding the use of counterfeit products or those administered in “non-medical settings.”
According to the Tennessee Department of Health, all four victims sought medical attention, and two were admitted to a hospital. The CDC stated that “laboratory-confirmed” occurrences of botulism after getting Botox injections are “rare.”
“Cosmetic injections should be an FDA-approved product, administered by licensed providers and in licensed settings,” a representative for the CDC stated.